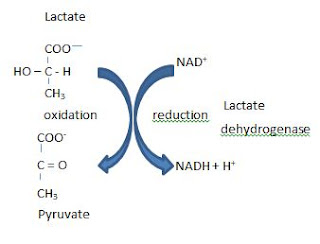
You all know passage of electricity through a metal wire means the passage of a stream of electrons. The passage of a stream of electrons through a conducting wire is equivalent to the passage of an electric current in the opposite direction.
Q1. What are the uses of an electric current ?
A1. 1. Obtaining the light energy (ex: Bulb)
2. Obtaining the heat energy (ex: heater)
3. Obtaining the Sound energy (ex radio)
Most of the chemical reactions involve simultaneous oxidation and reductions. Such a reaction is called a redox reaction. Electrons are generated in a redox reaction. But we cannot use them for any useful purpose. Can you think why?
Q2. Consider a Zn rod that has been immersed in a CuSO4 solution. What will be your observations ?
A zinc rod is immersed in a CuSO4 solution
A2. Here, a redox reaction takes place as we mentioned earlier. The following observations can be seen.
1. The color of the CuSO4 solution near the zinc rod fades
2. Deposition of a brown dust on the zinc rod
3. Dissolution of the zinc rod
Q3. Write down the oxidation and reduction half reactions and redox the reactions that occur in the example given above in Q2.
A3. Oxidation half reaction
Reduction half reaction
Redox Reaction
We Can explain the observations given above in Q2 with the help of the reactions given in Q3. Zn removes two electrons forming Zn 2+ and at the same place Cu2+ gains those two electrons depositing as Cu. Therefore you cannot use the electrons generated by the oxidation reaction to obtain a beneficial electric current.
If you planned to set up the happening of the redox reaction above, not in the same place but in two different places, then you will have to transfer the electrons removed by Zn, forming Zn 2+ towards Cu2+ to deposit as Cu. To achieve this you can use the apparatus given below.
Daniell Cell (incompleted)
Here the connection between two two systems has been established by using a conducting wire. Now you can transfer the electrons removed by Zn directly towards cu2+ through the wire and Cu rod.
Daniell Cell (completed) with the salt bridge
Daniell Cell (completed) with the prous wall
If you setup the apparatus as given above in the figure in Q7, You can use the current created to do external work. This type of an apparatus is called an electrochemical cell or a voltaic cell. We sometimes call it as a Galvanic cell where spontaneous redox reactions take place to produce electric current and do electric work. The compartment that can be separated from a salt bride or a porous wall is called an electrode or half cell. You can simply use the word cell to identify a Galvanic cell. There are two half cells in a Galvanic cell. In the above example Cu rod and CuSO4 solution forms one half cell. Zn rod and ZnSO4 solution forms the other half cell. We can use the word electrode to identify the metal rod in a half cell also. Quite often it is used to identify a half cell as well. The solution inside a half cell is called an electrolyte.
Generally the cell, that consist of Cu and Zn half cells is called the Daniell Cell. We can observe everything that happens in one system as discussed in Q3 in this Daniell cell also.
Operation of the salt bridge and porous wall
Consider the Daniell cell above. The Zn atoms in the Zn rod rod remove electrons and come to the ZnSO4 solution as Zn 2+ . The Cu2+ ions in the CuSO4 solution gain these electrons, that coming through the wire and Cu rod and deposit on the Cu rod as Cu. When this process take the no of Zn2+ ions in the ZnSO4 solution would be increased compared to SO42 ions. The no of Cu2+ ions in the CuSO4 solution would be decreased compared to SO42 ions. Therefore ZnSO4 solution would get slight positively charged and CuSO4 solution would get slight negatively charged. Finally an electrical imbalance is created in the cell and no further net flow of current will occur. To obtain a current from this electrochemical cell, the solutions must be electrically neutral. So that we use a salt bridge to establish this electrical neutrality. The Salt bridge consists with KCL. When the positive nature is increased in the ZnSO4 solution, Cl ions in the salt bridge migrate to the ZnSO4 solution and reduce the positive charge. When the negative nature is increased in the CuSO4 solution, K+ ions in the salt bridge migrate to the CuSO4 solution and reduce the negative charge. Therefore positive and negative ions go in opposite directions through a salt bridge.

Direction of the movement of ions inside the salt bridge
Now you all know that a porous wall is a simple device used to connect two half cells in a Galvanic cell. It is made out with microscopic holes. It separates two electrolyte solutions. So that ions could migrate between two solutions bringing electrical contact. consider the Daniell cell above. Cu2+ ions will start diffuse in to the ZnSO4 solution and Zn2+ ions will start to diffuse in to the CuSO4 solution when the two electrolyte solutions are connected at the porous wall. Therefore you can see a migration of ions through the wall. That means a current passes the circuit closed.
You all know passage of electricity through a metal wire means the passage of a stream of electrons. The passage of a stream of electrons through a conducting wire is equivalent to the passage of an electric current in the opposite direction.
Q1. What are the uses of an electric current ?
A1. 1. Obtaining the light energy (ex: Bulb)
2. Obtaining the heat energy (ex: heater)
3. Obtaining the Sound energy (ex radio)
Most of the chemical reactions involve simultaneous oxidation and reductions. Such a reaction is called a redox reaction. Electrons are generated in a redox reaction. But we cannot use them for any useful purpose. Can you think why?
Q2. Consider a Zn rod that has been immersed in a CuSO4 solution. What will be your observations ?
A zinc rod is immersed in a CuSO4 solution
A2. Here, a redox reaction takes place as we mentioned earlier. The following observations can be seen.
1. The color of the CuSO4 solution near the zinc rod fades
2. Deposition of a brown dust on the zinc rod
3. Dissolution of the zinc rod
Q3. Write down the oxidation and reduction half reactions and redox the reactions that occur in the example given above in Q2.
A3. Oxidation half reaction
Reduction half reaction
Redox Reaction
We Can explain the observations given above in Q2 with the help of the reactions given in Q3. Zn removes two electrons forming Zn 2+ and at the same place Cu2+ gains those two electrons depositing as Cu. Therefore you cannot use the electrons generated by the oxidation reaction to obtain a beneficial electric current.
If you planned to set up the happening of the redox reaction above, not in the same place but in two different places, then you will have to transfer the electrons removed by Zn, forming Zn 2+ towards Cu2+ to deposit as Cu. To achieve this you can use the apparatus given below.
Daniell Cell (incompleted)
Here the connection between two two systems has been established by using a conducting wire. Now you can transfer the electrons removed by Zn directly towards cu2+ through the wire and Cu rod.
Daniell Cell (completed) with the salt bridge
Daniell Cell (completed) with the prous wall
If you setup the apparatus as given above in the figure in Q7, You can use the current created to do external work. This type of an apparatus is called an electrochemical cell or a voltaic cell. We sometimes call it as a Galvanic cell where spontaneous redox reactions take place to produce electric current and do electric work. The compartment that can be separated from a salt bride or a porous wall is called an electrode or half cell. You can simply use the word cell to identify a Galvanic cell. There are two half cells in a Galvanic cell. In the above example Cu rod and CuSO4 solution forms one half cell. Zn rod and ZnSO4 solution forms the other half cell. We can use the word electrode to identify the metal rod in a half cell also. Quite often it is used to identify a half cell as well. The solution inside a half cell is called an electrolyte.
Generally the cell, that consist of Cu and Zn half cells is called the Daniell Cell. We can observe everything that happens in one system as discussed in Q3 in this Daniell cell also.
Operation of the salt bridge and porous wall
Consider the Daniell cell above. The Zn atoms in the Zn rod rod remove electrons and come to the ZnSO4 solution as Zn 2+ . The Cu2+ ions in the CuSO4 solution gain these electrons, that coming through the wire and Cu rod and deposit on the Cu rod as Cu. When this process take the no of Zn2+ ions in the ZnSO4 solution would be increased compared to SO42 ions. The no of Cu2+ ions in the CuSO4 solution would be decreased compared to SO42 ions. Therefore ZnSO4 solution would get slight positively charged and CuSO4 solution would get slight negatively charged. Finally an electrical imbalance is created in the cell and no further net flow of current will occur. To obtain a current from this electrochemical cell, the solutions must be electrically neutral. So that we use a salt bridge to establish this electrical neutrality. The Salt bridge consists with KCL. When the positive nature is increased in the ZnSO4 solution, Cl ions in the salt bridge migrate to the ZnSO4 solution and reduce the positive charge. When the negative nature is increased in the CuSO4 solution, K+ ions in the salt bridge migrate to the CuSO4 solution and reduce the negative charge. Therefore positive and negative ions go in opposite directions through a salt bridge.

Direction of the movement of ions inside the salt bridge
Now you all know that a porous wall is a simple device used to connect two half cells in a Galvanic cell. It is made out with microscopic holes. It separates two electrolyte solutions. So that ions could migrate between two solutions bringing electrical contact. consider the Daniell cell above. Cu2+ ions will start diffuse in to the ZnSO4 solution and Zn2+ ions will start to diffuse in to the CuSO4 solution when the two electrolyte solutions are connected at the porous wall. Therefore you can see a migration of ions through the wall. That means a current passes the circuit closed.
Posted at 2:46 AM | by
Unknown